 Loading... Please wait...
Loading... Please wait...Biocell Collagen
Product Description
Healthy aging via promoting both active joints and younger-looking skin
Retail $ 52.95
100 capsules
Our price $ 41.50
Joint Support
- Provides a multi-layered supports of healthy joint function by strengthening cartilage and replenishing synovial fluid
- Improves joint comfort and mobility leading to healthier daily activities
- Helps promote joint cushioning and lubrication due to HA and chondroitin sulfate content
- May help rebuild cartilage by stimulating the chondrocytes
Skin Support
- Reduces wrinkles and lines via counteracting both natural and photoaging processes.
- Reduces dryness/scaling via HA-mediated increase in skin hydration leading to enhanced turgidity and suppleness.
- Increases collagen content in the dermis supporting the tensile strength of the skin.
- Increase blood microcirculation in the face improving the skin texture
- Promote healthy skin aging and support the maintenance of glowing skin texture*
- May help improve the appearance of not only skin but also hair and nails.
| Since introducing BioCell Collagen in 1997, BioCell Technology has carried out various studies including four human clinical trials to prove its safety and efficacy. All the data obtained from these in-house studies has provided strong evidence that this innovative ingredient containing a naturally-occurring matrix of hydrolyzed collagen, LMW HA and chondroitin sulfate harbors a unique set of biological properties which are instrumental in comprehensively promoting both joint health and skin beauty. |
Toxicology Studies
Two acute and subchronic oral toxicity studies were conducted in rats to evaluate its safety. In the acute oral toxicity study, five males and five females of Sprague-Dawley rats were administered a single dose of 5000 mg of the test product per kg body weight and observed for 14 days. All animals survived and exhibited normal body weight gain throughout the study. Macroscopic necropsy examination conducted on day 15 revealed no gross pathological lesions in any of the animals.
In the subchronic study, rats were divided into four same-sex groups. Animals in each group were administered daily either 0, 30, 300 or 1000 mg of the test product per kg of body weight for over 90 days. All animals survived and showed no significant changes in their body weights and histopathology. The results from the two oral toxicity studies with male and female young adult rats indicated that BioCell Collagen was well tolerated at all four doses tested.
This study was published in Food and Chemical Toxicology in 2007.
Human Bioavailability Study
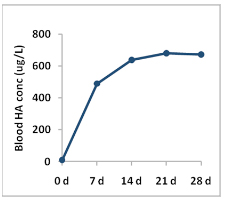
In this study, the peak absorption kinetics of HA from a single (1500 mg) dose of BioCell Collagen, and the 28-day bioavailability of HA and its metabolites were determined in a closed label study in the five normal healthy subjects. In the 36-hour peak absorption study active HA was found to be fast-absorbed into the bloodstream within several hours of ingestion increasing total HA concentration about 20-fold. HA level 24 fours after ingestion was 80 times as high as its baseline level. The 28-day steady state bioavailability study showed that ingestion of BioCell Collagen led to more than 60-fold increase in HA in human blood. These data suggested that the low molecular weight nature of hydrolyzed collagen, HA, and chondroitin sulfate contained in BioCell Collagen facilitated their fast and effective absorption into the circulation.
The details of the study are available upon request.
Multiple Human Clinical Trials
Clinical trials on joint benefits
There have been various clinical studies demonstrating the safety and beneficial effects of BioCell Collagen® on various joint discomforts.
- Clinical trial on subjective pain (1997)
The first human clinical trial studied the effects of ingesting 2 g of BioCell Collagen daily on subjective discomfort in 89 subjects suffering from various joint problems. This crossover, double blind study led to 89.9% (80 out of 89) of the subjects experiencing the reduction of joint discomfort.

-
A pilot clinical trial on managing various joint discomforts (2003)
This randomized, placebo-controlled, efficacy study examined 16 men and women with various joint discomforts with mobility restriction, revealing significant efficacy as well as safety of BioCell Collagen in relieving joint discomforts. This 8-week long trial showed that daily intake of 2 grams of BioCell Collagen led to as much as 40% of improvements, which was significantly superior to the group receiving placebo supplements.
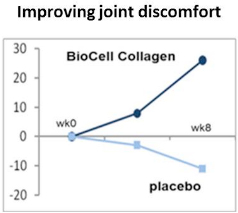
The outcome of the study was presented in the International conference of Experimental Biology 2004, Washington D.C.
-
A confirmatory clinical trial on chronic joint condition (2010)
A larger trial involving 80 humans with moderate-to-severe joint discomforts replicated the earlier findings demonstrating its safety and efficacy in improving joint discomforts and thereby in enhancing various physical activities of daily life.
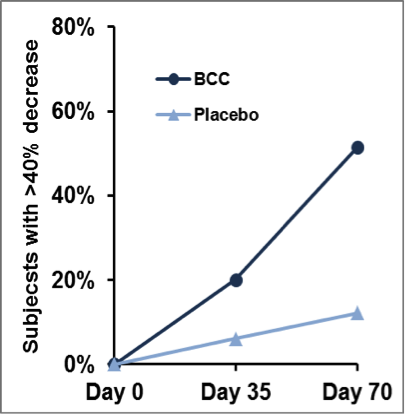
The outcome of this study was published in the Journal of Agricultural and Food Chemistry in 2012.
Human Skin Study – Beauty- From-Within Effects
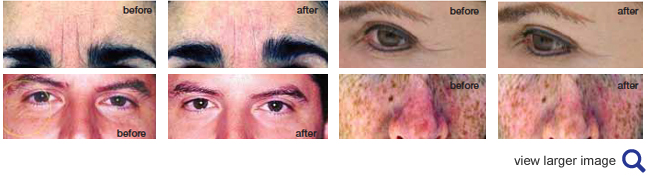
In 2011, BioCell Technology performed a ground-breaking human skin study and demonstrated that daily ingestion of BioCell Collagen for 12 weeks led to a significant reduction of facial lines and wrinkles together with enhancement of hydration and stimulation of collagen biosynthesis likely from dermal fibroblasts. It also led to an increase in hemoglobin in the facial skin tissue suggesting enhanced microcirculation of blood in the dermis. The majority of the study participants experienced improved skin texture as well as enhanced moisture and reduced scaling in their face.
The top-line outcome of this study was announced here and its details were submitted for publication in a peer-reviewed journal.
Hyaluronidase Inhibition Study
What is unique and intriguing with BioCell Collagen is its inhibitory activity of hyaluronidase. HA is present in virtually all tissues and bodily fluids while one third of HA is turned over daily by coordinated action of HA synthases and hyaluronidases. Our in vitro study showed that hyaluronidase was specifically inhibited by BioCell Collagen in a dose-dependent fashion. Given that hyaluronidase-mediated degradation of HA is implicated in various aging-associated physiological conditions, BioCell Collagen’s property of inhibiting hyaluronidase may have a profound implication especially in terms of aging processes which takes place in the joint and the skin. This data suggested that BioCell Collagen had multiple biological properties that can be harnessed to maintain the integrity and amount of HA which is essential for active joint and younger-looking skin.
BioCell Collagen is non-GMO and free of gluten, soy, shellfish, fish, egg, milk, peanuts and sugar.
Product Videos
-
 Not All Collagen Supplements Are Created EqualAlternative health expert and author Bryce Wylde interviews Ha...
Not All Collagen Supplements Are Created EqualAlternative health expert and author Bryce Wylde interviews Ha...







 Our newsletter
Our newsletter